*in progress*
With intermittent frequency I read trip reports, articles, social media comment threads or whatever from mountaineers and climbers who go to altitude and say many partially correct things about oxygen and altitude with a smaller number of completely wrong things mixed in.
Every so often my frustration boils over and I write a comment or reply or DM to someone and try to explain things and correct their misapprehensions. It's happened enough times that I figured I should write down the most salient aspects of respiratory physiology at altitude in a way that (hopefully) makes sense to mountaineers and climbers.
Background
As background, I'm a pediatric critical care physician and an amateur mountaineer. The former gives me a lot of expertise in respiratory physiology; as to the latter, I haven't done anything noteworthy but I've done enough rapid ascents from sea level to 4000 meters to have experienced AMS and have spent a lot of time cavorting the mountains.
I can't promise that anything in this writeup will help you climb a mountain but I hope that it will help you understand what's going on in your own body at altitude and maybe help you make plans and sound decisions.
Also, I'm a barbarian American and our weird meteorological pressure measurements have found their way into our physiology, so my apologies if you've stumbled upon this writeup from the enlightened world. There are plenty of calculators to turn mmHg into kPa if you wish. I will probably also switch randomly between feet and meters when referring to altitude as different domains (climbing, meteorology, etc) have different norms for me.
For the most part I'm going to write this without putting in a ton of references because, as anyone who's ever done academic writing can attest, it's an exorbitant amount of work and I trust that all of my readers can operate a search engine. For things that are esoteric, hard to dig up, or from whence I've borrowed images I'll try and put in a link. Much of the primary literature on this stuff pre-dates digital publication so has been retroactively scanned or is otherwise a pain to get ahold of beyond abstracts.
With that throat clearing aside, one law of physics that will come up over and over again throughout this discussion that is very important (and thankfully simply and intuitive):
Dalton's Law says that in any mixture of gasses the pressure that each exerts is proportional to its concentration and all of the proportions add up to the total. Putting this into layman's terms is really easy: all of the parts make up the whole and all of the parts take up as much space as their proportion.
Ambient Air and Barometric Pressure:
Below the tropopause (~36,000 ft) air is well mixed by a bunch of different physical phenomena and its composition is the same over the whole surface of the earth (local pollution notwithstanding) and 20.947% of the molecules in that air are oxygen molecules. Just round it to 21% for your own and everyone else's sanity.
As we ascend in altitude the barometric pressure decreases. This is because the atmosphere above us has substance and mass and as we go up, there is less of it above us. Less stuff above getting pulled down by gravity means less pressure. There are a couple of mathematical models that explain how much the pressure decreases as the altitude increases, if you find yourself worrying about this: choose the
model atmosphere equation (
not the standard atmosphere equation) as its output matches measured data much better.
As this barometric pressure decreases it doesn't change the composition of the atmospheric air (20.947% of the molecules in the air are still oxygen) but it does mean there are fewer molecules in the air -- including oxygen. This means that there are fewer oxygen molecules inside of our lungs (since the volume of our lungs doesn't change as we ascend in altitude) available for our body to absorb.
While altitude is, by far, the largest factor in barometric pressure changes that a climber may encounter, there are others which bear mentioning.
Latitude, Temperature, Weather
For a bunch of complicated physics reasons there is a bulge in the atmosphere over the equator which has the effect of increasing the barometric pressure the closer one gets to the equator (when compared to the same altitude nearer the poles) because the troposphere is about twice as thick (5km vs 10km).
Anyone with a passing familiarity with meteorology will recognize that it is usually discussed in terms of high and low pressure regions -- the interaction between them being what gives us weather. Depending on where you live and its usual weather patterns a "strong" high or low pressure system will usually deviate from normal by about 25 millibars (domain specific units are very annoying in this sort of cross discipline discussion… 25 millibar ≈ 19 mmHg). As we'll see later a 20 mmHg change in barometric pressure can have dramatic effects on our body's ability to absorb oxygen.
 |
| Pressure Map (via windy.com on 20 August 2023). Numbers are mmHg. Note the tropical storm and its low pressure center off the coast of California compared to the high pressure center south of Alaska. |
There is also a diurnal (ie day-night) fluctuation to barometric pressure which varies by latitude but is generally small enough to be ignored (the shift is +/- 2.5 millibar (≈1.9 mmHg) at most).
A lot of work has been done developing (
for example) mathematical models for the interaction of temperature, latitude, and altitude to determine the difference between physical height and pressure height of a variety of summits. This work builds upon experimental data that has confirmed similar effects. In general the further away a peak is from the equator the more dramatic the difference between physical and pressure heights will be; though, these effects rarely lead to a difference of more than 200-300m to; moreover, since the great ranges mostly lie near the equator this effect usually serves to lower a summit (in pressure terms) rather than raise it. Denali is the big exception with winter conditions generally making the summit ~600m taller in pressure height vs physical height. I'm not aware of any data for Mount Vinson (
calculations exist -- though these particular ones use the standard atmosphere rather than the model atmosphere) or other more polar peaks, but I'm also not aware of winter ascents of those peaks.
 |
| Physical altitude vs pressure altitude values calculated (from the standard atmosphere model) and extrapolated from distant temperature measurements. (Source) |
Respiratory Physiology
Now we need to move on to how these pressure differences affect our respiratory physiology and why very small differences in barometric pressure can make such enormous differences to human physiology.
Our breathing serves two purposes: 1) to move oxygen molecules from the atmosphere into our lungs (the alveoli within the lung is where gas exchange happens but mostly I will just use the term lung to refer to the part of the lung where gas exchange occurs) and 2) to move carbon dioxide molecules produced by our metabolism out into the atmosphere. Per the title of this article we're talking about oxygen physiology, but it turns out that carbon dioxide matters as well since there's a lot more of it in the air we breathe out than in the atmosphere and it takes up space, so it'll come up later.
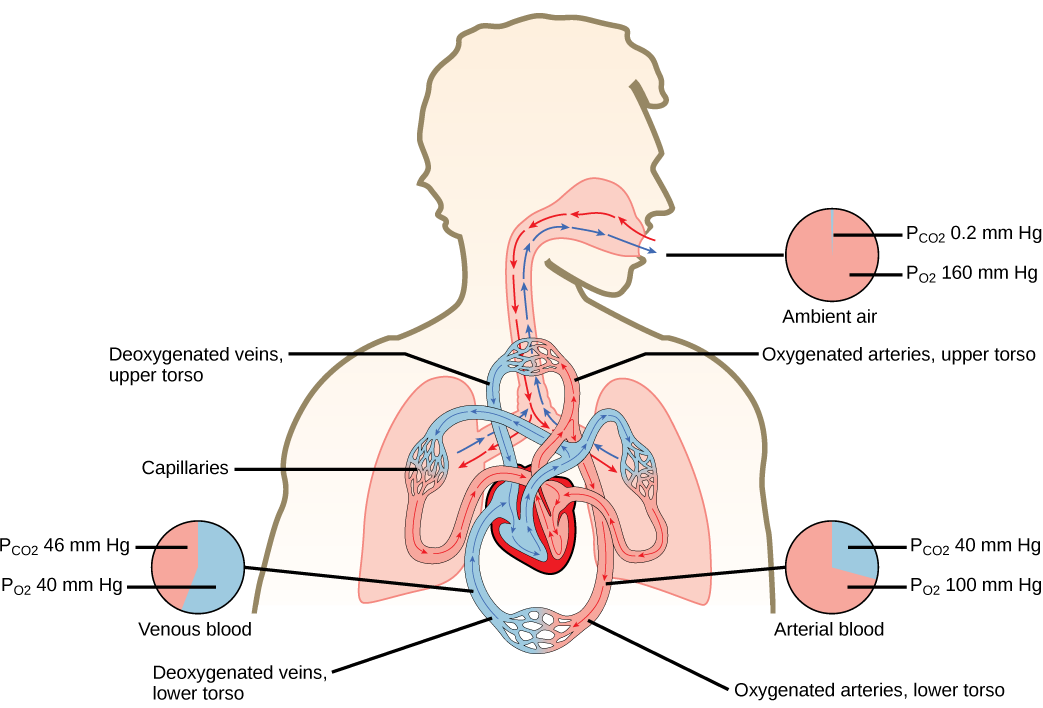 |
| Respiratory system overview (pressures in individuals at rest, at sea level and healthy). |
Oxygen Absorption and Carrying
The main way that our body gets and transports oxygen from the lung is by using hemoglobin which is an oxygen transporter molecule that lives within our red blood cells. Without hemoglobin we wouldn't be able to deliver enough oxygen to our brain or organs. There's even an equation that tells us how many mL of oxygen is in each dL of blood:
Arterial Oxygen Content = (1.34 x Hemoglobin x Percent Arterial Oxygen Saturation) + (Pressure of dissolved oxygen in arterial blood * 0.003)
 |
| Note that almost all oxygen is carried on hemoglobin molecules. (source) |
Whether or not hemoglobin molecules with carry or release oxygen molecules is determined (mainly) by the pressure of oxygen in your blood. This effect is not linear and very non-intuitive. Very small changes in oxygen pressure can make comparatively large changes in the amount of hemoglobin that is carrying oxygen. More on this later.
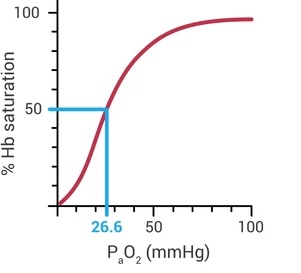 |
| Standard oxyhemoglobin dissociation curve under usual physiologic conditions. |
When we breathe our nose, mouth, throat, and trachea heat and saturate the air we breathe with water before it reaches our lungs. This is necessary because the tissue in our lungs is very fragile and cold, arid air is quite irritating to the body. It's a problem for people at altitude because of Dalton's law though -- all of that water vapor in the air displaces other gasses, including oxygen.
Similar to the calculation we can do to determine how much oxygen is in someone's blood we can also calculate the amount of oxygen in a person's lung that is available for the body to absorb and use:
Oxygen pressure in the lung = [(Barometric Pressure - Water Vapor Pressure) * Oxygen %] - [Blood CO2 level / O2-CO2 exchange constant]
I promise that the important concepts in this equation are much more straightforward than the complexity of the equation would make it seem. Let's take the terms one at a time and combine them with things already laid out above to bring some important considerations forward.
Before we move on to breaking down the components of the equation, we need to note that its output tells us the maximum amount of oxygen pressure inside the air sacs of our lung. For a variety of
physics and physiology reasons we cannot achieve arterial oxygen amounts that are equivalent;
there will always be at least a small decrement between this equations's output and our blood's oxygen pressure.
Discussing the first half of the equation: the FiO2 is the same for everywhere on Earth (~21%). The atmospheric (aka barometric) pressure is what we've talked about at length above that will fluctuate with altitude, season, weather, time of day, and so forth. The water vapor pressure inside our lung will always be the same (47 mmHg) because we heat all our inspired air to our body temperature and fully saturate it with water, thus it represents a fixed loss of available atmospheric pressure. Looking at this equation it becomes clear why supplemental oxygen can make such a big difference when climbing at very high altitudes. Anything that moves the 21% number upward will result in a much larger amount of oxygen pressure within the lung (ie able to be absorbed into the blood stream).
As to the second half of the equation we'll start with RQ. Conceptually RQ represents the ratio of carbon dioxide molecules we release to oxygen molecules that we absorb. For nearly all humans (though not infants) eating normal diets (including what people generally consume at high altitudes) it is generally approximated at 0.8 (a variety of studies using direct measurement of human subjects at pressures below 350 mmHg have found a mean value of 0.82). If you want further information Wikipedia's
explanation is sound.
Carbon Dioxide (ie Ventilation)
A language note before we dive in: the process of moving oxygen into our body is referred to by physiologists as oxygenation which is simple enough; but the process of getting rid of carbon dioxide from our body is referred to as ventilation. Thus when we someone is discussed as hyperventilating it means they are breathing their carbon dioxide levels down below normal; someone who is hypoventilating is allowing their carbon dioxide levels to accumulate or rise beyond where the body wants them to be.
As mentioned conceptually and now mathematically, the amount of carbon dioxide in our blood (and subsequently in our exhaled breath) takes up space that could otherwise be occupied by oxygen. Our body regulates its carbon dioxide pressure within a very narrow range (the normal pressure of CO2 in our arterial blood is 40 mmHg +/- 5) because it is tied to many aspects of our physiology such as: the blood pressure within our lungs, the amount of blood flow to our brain, and the ability of muscles to contract and relax normally are all intricately linked to our carbon dioxide concentration directly or indirectly.
As a brief aside, carbon dioxide is the reason you cannot hold your breath very long. At sea level most healthy adults can go 4-6 minutes without breathing before their oxygen levels (measured by hemoglobin saturation) will start to fall, yet most people can only hold their breath for 1-2 minutes. The reason for this is the build up of carbon dioxide which directly stimulates our brain to start breathing. In fact, if one holds their breath long enough, through the excruciating discomfort, the brainstem will force the diaphragm to start contracting despite a person willing it not to.
Perhaps obvious, but worth stating clearly: as holding our breath causes carbon dioxide to accumulate, breathing more rapidly (or more deeply, or both) causes our carbon dioxide level to decrease from normal. If you do this long enough a variety of things related to the physiological connections I mentioned above will start to occur (you'll feel tingly, your fingers and toes may spasm, you'll get a headache, you might get nauseous).
I've avoided doing math as much as I can in this article (despite the presence of many equations), but working through the alveolar gas equation will illustrate the importance of carbon dioxide at extreme altitudes in a way that words cannot (recall that the result of these calculations is the oxygen pressure available to our body):
The alveolar gas equation at sea level (all pressures in mmHg):
On the summit of Mt. Everest (8849 meters):
On the summit of Mt. Rainier (4392 meters):
Put another way: if humans do not hyperventilate on the summit of Mt. Everest, there cannot be any oxygen in their lungs (though this is sort of a paradox, since if there's no oxygen in their lung they'd be dead and they wouldn't be producing carbon dioxide…). Even on the summit of Mt. Rainier an oxygen pressure of 32.5 mmHg without hyperventilation would mean a climber has a hemoglobin saturation of only ~60% (see the oxyhemoglobin chart above)!
Now that we've worked through the math we can talk about acclimatization, or to put it another way: how our body can fiddle with some things to overcome these unalterable constraints of physics.